Mesothelioma Advocates Recommend Patients Receiving Immunotherapy Treatment Get COVID-19 Vaccine
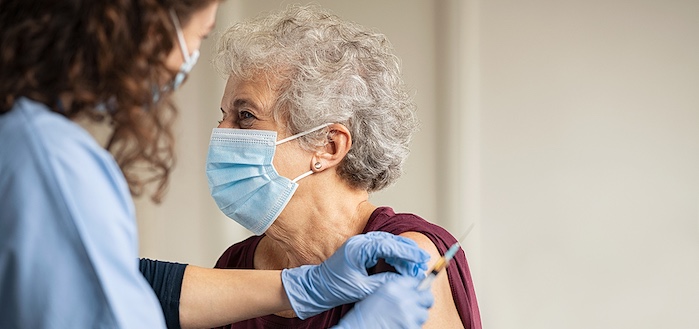
The COVID-19 pandemic changed life in the United States about one year ago this week. Since then, scientists have worked tirelessly to develop vaccines to protect against the disease. For mesothelioma cancer patients, the risk of contracting COVID-19 can be especially life-threatening.
As vaccine appointments become available, cancer patients and survivors may wonder if the vaccine is right for them. The Mesothelioma Applied Research Foundation (Meso Foundation) has recently recommended mesothelioma patients undergoing immunotherapy receive the COVID-19 vaccine.
Request a Free 2024 Mesothelioma Guide
Should Cancer Patients Get the COVID-19 Vaccine?
Mesothelioma patients, and all cancer patients, are considered high risk for serious COVID-19 complications. As a result of the potential for complications, many mesothelioma patients may be eager to receive the COVID-19 vaccine.
The vaccine has multiple benefits for the general public:
- It may prevent contracting COVID-19.
- If a person does catch the virus, the vaccine may prevent its most serious effects.
For cancer patients and survivors, the decision to receive the vaccine may be more nuanced than an otherwise healthy person.
Patients receiving cancer treatment should talk to their doctors about getting the vaccine. There may be potential interactions between the vaccine and their cancer treatments. Mesothelioma cancer survivors may also have lasting side effects from cancer treatment. These effects may impact the risks and benefits related to the COVID-19 vaccine.
Communication between patients and medical staff can ensure the safety of the patient.
Are Cancer Patients Eligible for the Vaccine Everywhere?
COVID-19 vaccine eligibility varies by state. The Centers for Disease Control and Prevention (CDC) provided recommendations for who should initially receive the vaccine. However, states are not required to follow these recommendations.
Cancer patients were not in the first group recommended for the vaccine by the CDC. However, cancer is on the CDC’s list of underlying medical conditions leading to increased risk for severe COVID-19 illness. As a result, cancer patients may be eligible for the vaccine in some states.
Individuals should check their local government’s website for the most up-to-date eligibility requirements for their area. A local physician may also assist in determining if and when patients may receive the vaccine.
Agencies Recommending the COVID-19 Vaccine for Cancer Patients
In general, medical agencies have recommended cancer patients receive the COVID-19 vaccine. However, each person’s case is different.
For example, the American Society of Clinical Oncology (ASCO) and MD Anderson Cancer Institute have recommended the COVID-19 vaccine for:
- Cancer patients in active treatment
- Individuals with a history of cancer
- Cancer survivors
In general, medical experts are suggesting individuals eligible for the vaccine receive it, as long as there are no contraindications.
According to the CDC, the COVID-19 vaccines from Pfizer-BioNTech and Moderna have the following contraindications:
- A severe allergic reaction to a previous dose of the vaccine or its components
- An immediate allergic reaction to the vaccine or its components
- A diagnosed allergy to PEG (polyethylene glycol) and/or polysorbate (a common ingredient in childhood vaccines, such as DTaP)
Recently, a third COVID-19 vaccine, the Janssen vaccine, was given emergency approval. According to the CDC, it has the following contraindication:
- A known history of a severe allergic reaction to a previous dose of the vaccine or its components
COVID-19 Vaccination Recommended for Patients Undergoing Immunotherapy
The Meso Foundation has specifically recommended the COVID-19 vaccination for mesothelioma patients treated with immunotherapy. This guidance aligns with the Society for Immunotherapy of Cancer (SITC).
The SITC recommends:
- All cancer patients receiving immunotherapy as standard care or through a clinical trial get the vaccine, as long as there are no contraindications
- Patients do not receive experimental or non-FDA-approved vaccines outside of clinical trials
The SITC has also stated due to limited data, the interactions between immunotherapy treatments and the COVID-19 vaccine are currently not known.
Additionally, the agency has said immunosuppressed cancer patients may not have the desired immune response to the vaccine. As a result, these patients may need booster shots. For example, patients treated with chemotherapy may fall into the immunosuppressed category.
Should Other Mesothelioma Patients Receive the COVID-19 Vaccine?
Currently, no medical organizations have made a COVID-19 vaccination recommendation for the broader mesothelioma community.
Mesothelioma patients and survivors without specific contraindications may benefit from receiving the COVID-19 vaccine. Patients should talk to a mesothelioma specialist before any vaccinations. A doctor familiar with their medical history and treatments can explain potential benefits and risks.